Editor’s note: this guest post was contributed by ten leading psychiatric geneticists (see author list at the end of the post) in response to the headline-grabbing claims of a recent paper claiming to have identified eight genetic sub-types of schizophrenia. Similar text has also been posted on PubMed Commons and elsewhere. [DM]
In a study published on September 15, Arnedo et al. asserted that schizophrenia is a heterogeneous group of disorders underpinned by different genetic networks mapping to differing sets of clinical symptoms. As a result of their analyses, Arnedo et al. have made remarkable and perhaps unprecedented claims regarding their capacity to subtype schizophrenia. This paper has received considerable media attention. One claim features in many media reports, that schizophrenia can be delineated into “8 types”. If these claims are replicable and consistent, then the work reported in this paper would constitute an important advance into our knowledge of the etiology of schizophrenia.
Unfortunately, these extraordinary claims are not justified by the data and analyses presented. Their claims are based upon complex (and we believe flawed) analyses that are said to reveal links between clusters of clinical data points and patterns of data generated by looking at millions of genetic data points. Instead of the complexities favored by Arnedo et al., there are far simpler alternative explanations for the patterns they observed. We believe that the authors have not excluded important alternative explanations – if we are correct, then the major conclusions of this paper are invalidated.
Continue reading ‘Eight types of schizophrenia? Not so fast…’
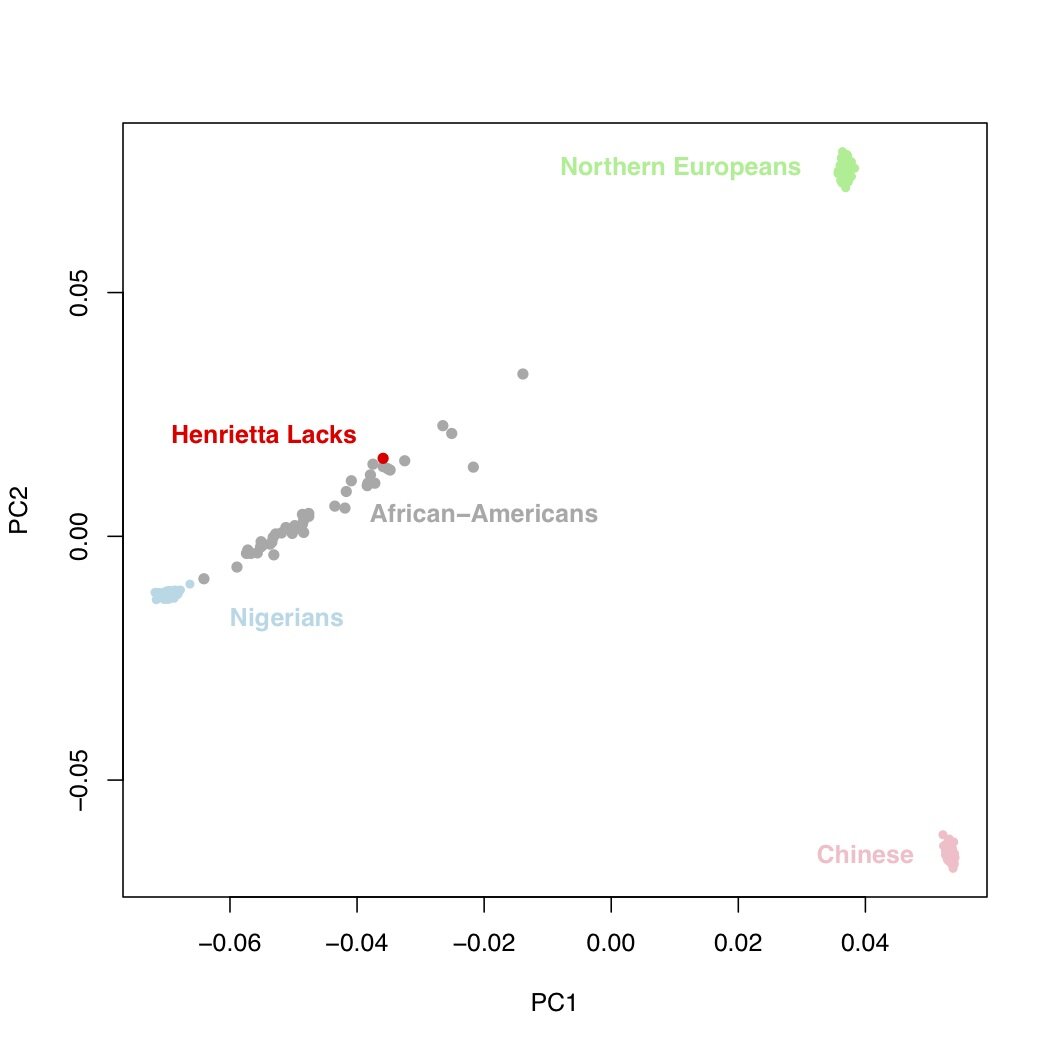
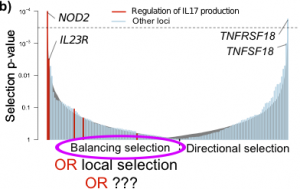 As I
As I 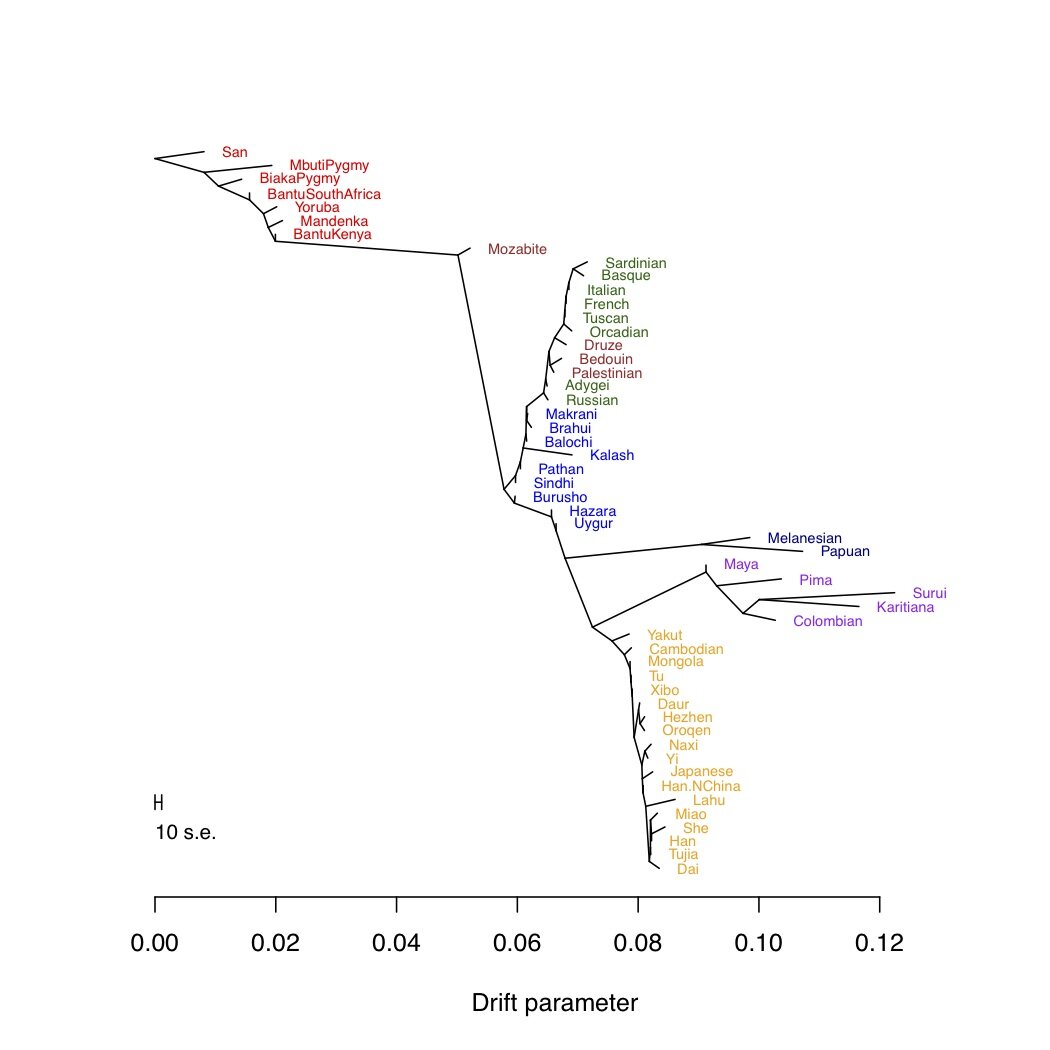

 RSS
RSS Twitter
Twitter
Recent Comments