 Dr Anna Middleton is an Ethics Researcher and Registered Genetic Counsellor, based at the Wellcome Trust Sanger Institute. She leads the ethics component of the Deciphering Developmental Disorders study, a collaborative project involving WTSI and the 23 National Health Service Regional Clinical Genetics Services in the UK. This project involves searching for the genetic cause of developmental disorders, using array-CGH, SNP genotyping and exome sequencing, in ~12,000 children in the UK who currently have no genetic diagnosis.
Dr Anna Middleton is an Ethics Researcher and Registered Genetic Counsellor, based at the Wellcome Trust Sanger Institute. She leads the ethics component of the Deciphering Developmental Disorders study, a collaborative project involving WTSI and the 23 National Health Service Regional Clinical Genetics Services in the UK. This project involves searching for the genetic cause of developmental disorders, using array-CGH, SNP genotyping and exome sequencing, in ~12,000 children in the UK who currently have no genetic diagnosis.
One of the issues raised by this, and many other research projects, is what should happen to ‘incidental’ findings, i.e. potentially interesting results from genomic analyses that are not directly related to the condition under study. Here Anna discusses the research she is conducting on this topic as part of the DDD study, and provides a link to the DDD Genomethics survey where you can share your own views (I should also disclose here that both Caroline and I also work on the DDD study).[KIM]
Whole genome studies have the ability to produce enormous volumes of valuable data for individuals who take part in research. However, as a consequence of analysing all 20,000+ genes, whole genome studies unavoidably involve the discovery of health related information that may have actual clinical significance for the research participant. Some of this will be considered a ‘pertinent finding’, i.e. directly related to the phenotype under study (e.g. the child’s developmental disorder); some of this will be considered an ‘incidental or secondary finding’ in that it is not directly linked to the phenotype under study or the research question that the genomic researchers are trying to answer.
Continue reading ‘Ethics and Genomic Research: ‘Genomethics’’
 I have no strong family history of any disease, despite having 7 blood aunts and uncles and countless cousins. So when I sent my spit off to 23andMe at the start of the Genomes Unzipped project, I was expecting something very similar to
I have no strong family history of any disease, despite having 7 blood aunts and uncles and countless cousins. So when I sent my spit off to 23andMe at the start of the Genomes Unzipped project, I was expecting something very similar to  To celebrate 10 years since the back-to-back publications of complete human genomes in Science and Nature, Science has published series of articles looking back at the last 10 years of genomics, and forward to the future. The article contains short essays from Francis Collins and Craig Venter, the former talking about some of the successes of medical sequencing (including giving a name and photograph to the exome-sequenced IBD patient I
To celebrate 10 years since the back-to-back publications of complete human genomes in Science and Nature, Science has published series of articles looking back at the last 10 years of genomics, and forward to the future. The article contains short essays from Francis Collins and Craig Venter, the former talking about some of the successes of medical sequencing (including giving a name and photograph to the exome-sequenced IBD patient I  Of particular interest is an article by Eliot Marshall on why genomics hasn’t yet had a large effect on medical practice, and what needs to be done to allow the genomic revolution to trickle into medical care. He argues that scientists and doctors need to meet each other half way; scientists need to focus more on showing the direct clinical utility of genomics, whereas doctors need to be more ready to accept new technologies and discoveries, and adapt the way they practice medicine to make full use of them. [LJ]
Of particular interest is an article by Eliot Marshall on why genomics hasn’t yet had a large effect on medical practice, and what needs to be done to allow the genomic revolution to trickle into medical care. He argues that scientists and doctors need to meet each other half way; scientists need to focus more on showing the direct clinical utility of genomics, whereas doctors need to be more ready to accept new technologies and discoveries, and adapt the way they practice medicine to make full use of them. [LJ] Illumina CEO Jay Flatley announced that an upgrade to their HiSeq 2000 platform expected this spring will allow users to generate 600 gigabases of sequence (the equivalent of 5 high quality human genomes) per one-week run of the machine. This would essentially double the current throughput of the platform and propel Illumina even further ahead in the arms race of delivering vast quantities of low cost sequence data. [JCB]
Illumina CEO Jay Flatley announced that an upgrade to their HiSeq 2000 platform expected this spring will allow users to generate 600 gigabases of sequence (the equivalent of 5 high quality human genomes) per one-week run of the machine. This would essentially double the current throughput of the platform and propel Illumina even further ahead in the arms race of delivering vast quantities of low cost sequence data. [JCB]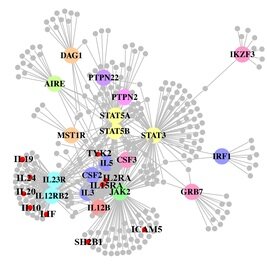 A paper out in PLoS Genetics this week takes a step towards using genome-wide association data to reconstruct functional pathways. Using protein-protein interaction data and tissue-specific expression data, the authors reconstruct biochemical pathways that underlie various diseases, by looking for variants that interact with genes in GWAS regions. These networks can then tell us about what systems are disrupted by GWAS variants as a whole, as well as identifying potential drug targets. The figure to the right shows the network constructed for Crohn’s disease; large colored circles are genes in GWAS loci, small grey circles are other genes in the network they constructed. As an interesting side note, the GWAS variants were taken from a 2008 study; since then, we have published a new meta-analysis, which implicated a lot of new regions. 10 genes in these regions, marked as small red circles on the figure, were also in the disease network. [LJ]
A paper out in PLoS Genetics this week takes a step towards using genome-wide association data to reconstruct functional pathways. Using protein-protein interaction data and tissue-specific expression data, the authors reconstruct biochemical pathways that underlie various diseases, by looking for variants that interact with genes in GWAS regions. These networks can then tell us about what systems are disrupted by GWAS variants as a whole, as well as identifying potential drug targets. The figure to the right shows the network constructed for Crohn’s disease; large colored circles are genes in GWAS loci, small grey circles are other genes in the network they constructed. As an interesting side note, the GWAS variants were taken from a 2008 study; since then, we have published a new meta-analysis, which implicated a lot of new regions. 10 genes in these regions, marked as small red circles on the figure, were also in the disease network. [LJ] A couple of interesting articles this week on the Personal Genome Project and public genomics in general. Mark Henderson at the Times has an opinion piece (behind a paywall, I’m afraid) about Misha Angrist‘s book Here Is A Human Being (see also this review from The Intersection), and in the Duke Magazine Mary Carmichael has an in-depth feature on the work of George Church, with some interesting history of the early days of the PGP.
A couple of interesting articles this week on the Personal Genome Project and public genomics in general. Mark Henderson at the Times has an opinion piece (behind a paywall, I’m afraid) about Misha Angrist‘s book Here Is A Human Being (see also this review from The Intersection), and in the Duke Magazine Mary Carmichael has an in-depth feature on the work of George Church, with some interesting history of the early days of the PGP. RSS
RSS Twitter
Twitter
Recent Comments